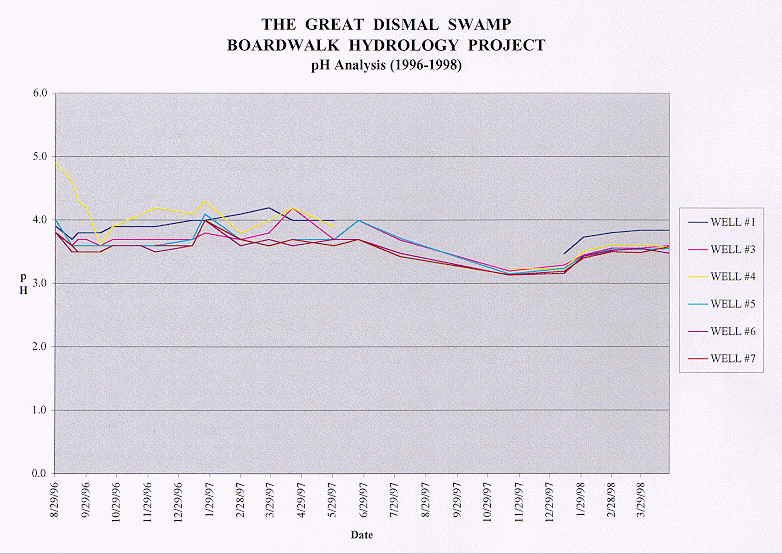
The pH of the water in the Great Dismal Swamp is fairly consistent throughout the year ranging on the average between 3.5 to 4.0 mg/L. This acidic water is affected somewhat from extreme periods of precipitation which raises the pH level making the water closer to being neutral (7.0). This can be seen by comparing the charts displaying pH, dissolved oxygen, and water table pulsing. For instance, on the pH graph a small spike can be seen between December 29, 1996 and January 29, 1997. Spikes also show up on the dissolved oxygen and water table graphs at the same time period suggesting precipitation had fallen near the date the water samples were taken.
Several possible causes can be given to as why the water in the Great Dismal Swamp is so acidic. One definite cause of the acidity is that the water has tannic acid in it. Anyone that goes out into the swamp and looks at the water can see the brownish-tan coloration that comes from tannic acid. The acid forms from the tannins found in plants that have reacted in the environment.
Another explanation of the water's acidity has to do with carbon dioxide. Often where you find carbon dioxide and organic material, you will also find carbonic acid.
Carbonic acid (H2CO3) is produced by the oxidation or organic matter by microbes to CO2. In other words, representing organic matter as CH2O:
CH20 + O2 --> CO2 + H20
This CO2 then combines with water to form carbonic acid, which further partly dissociates (it is a weak acid) to H+ and HCO3-:
CO2 + H20 --> H2CO3 H2CO3 --> H+ + HCO3-
(Berner 1987).
Continuing with possible causes of the swamp's acidity, it came in to consideration that sulfuric acid may also play a part in the water's pH if any sulfur could be found. According to Kirk's The Great Dismal Swamp, sulfur is in the swamp's water (Kirk 1979). "Sulfuric acid (H2SO4) is produced by the bacterially catalyzed oxidation of sulfide minerals..." (Berner 1987).
Other sources of the water's low pH level may be nitric acid (which forms from the nitrate in the soil and water) and possibly to some extent rainwater which generally has a pH ranging from 4 to 6 (Berner 1987).
